Governance Documentation
A link to the folder containing all the governance documentation can be found here. This includes the following documents:
- ACT Network Operating Procedures
- ACT Standard Operating Procedures
- NCATS ACT Governance
- ACT Terms of Query Access
Regulatory Documentation
A link to the folder containing all the regulatory documentation can be found here. This includes the following documents:
- NCATS ACT i2b2 SHRINE Repository Protocol
- NCATS Regulatory Guidance Overview
- NCATS Non-Human Subjects Research Activity
ACT Governance Structure
The ACT governance structure includes required reporting to the National Center for Advancing Translational Sciences (NCATS) through the ACT PI Group, which is made up of four Principal Investigators:
- Steven Reis, University of Pittsburgh
- Gary Firestein, UCSD
- Robert Toto, UTSW
- Lee Nadler, Harvard
The ACT Executive Committee reports to the ACT PI Group and provides oversight for the project. Reporting to the ACT Executive Committee are five work groups, as outlined in the figure below:
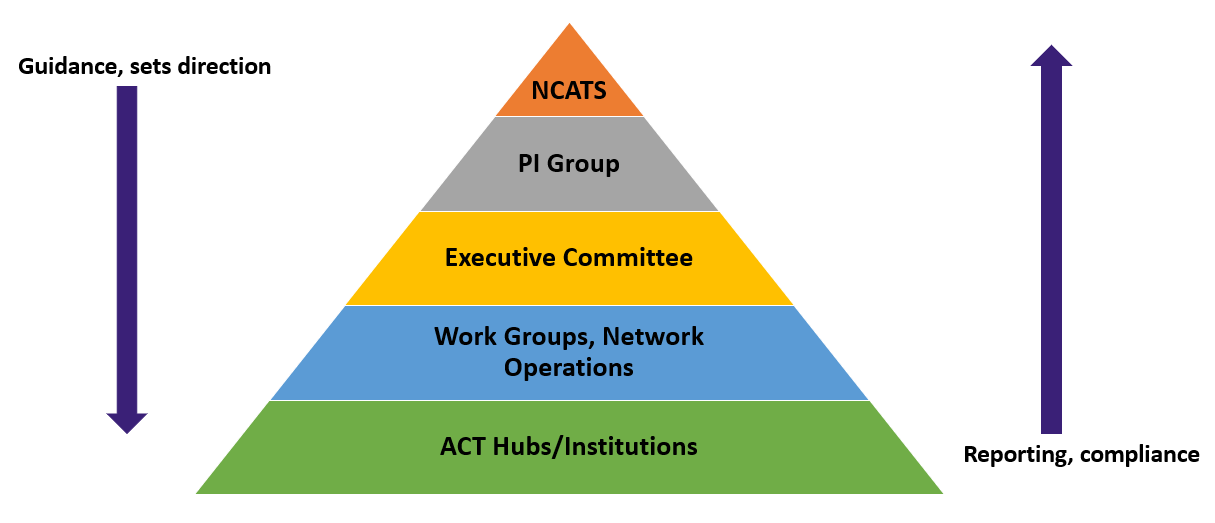
ACT Site Responsibilities
Each ACT site is expected to adhere to the following:
- Stay current on software versioning as guided by ACT Network Operations
- Ensure integrity of data use by regularly monitoring local query activity
- Adhere to expected uptime policies and work through issues in a timely manner
- Refresh data on at least a monthly basis
ACT governance also outlines three standard operating procedures for ACT and ACT sites, listed below:
SOP 1: Adding New or Modifying Existing Data Domains. The Data Harmonization work group defined a set of data domains (e.g. demographics, diagnoses, procedures) and standards for representing data in these domains. As ACT continues to evolve, there is the need to modify current domains and add new domains. This SOP lists the process required to suggest additions and modifications to the ACT data domains.
SOP 2: Process for User Registration and Management. This SOP ensures that the ACT Network is accessed only by individuals authorized by a participating ACT site. User registration and management is handled locally at each ACT site.
SOP 3: Monitoring and Auditing. To ensure ACT achieves its goals and participating sites operate in accordance with ACT governance, query activity will be periodically audited by local site Data Stewards. Only authorized individuals will have access to information that can identify the user. Network usage metrics will also be collected on a regular basis, but will not include user identifying information.
HIPAA & IRB Information
The ACT Network only shares aggregate patient counts, and never shares (or accesses) patient-level data. Each site monitors and reports on local usage of the ACT Network to ensure compliance with the ACT Data Use Agreement.
Part of the ACT implementation process at each site involves applying for approval from the site’s IRB. No additional study-specific IRB approval is needed for investigators using ACT.
Citing ACT
Publications based on research using the ACT Network must cite the NCATS ACT grant: “This project was supported by the National Institutes of Health through grant UL1TR000005.” Publications should also cite the appropriate CTSA hub grant numbers from participating institutions.
Any Intellectual Property derived from use of the ACT Network must cite the NCATS ACT grant: “This project was supported by the National Institutes of Health through grant UL1TR000005.”
Publications in which data source partners (hospitals) are to be identified by name will be reviewed for use of name only by each identified hospital prior to submission of a manuscript. At no time will specific participating data source partners be named unless explicitly approved by the data partner. Such approval must be requested and received in writing between the requestor and the Senior Vice President of Research, the Chief Information Officer, or their respective designee. Any entity (e.g., hospital) that does not agree to be identified by name as a data source will be instead identified as a “CTSA-affiliated hospital.”